The YIN Lab Research
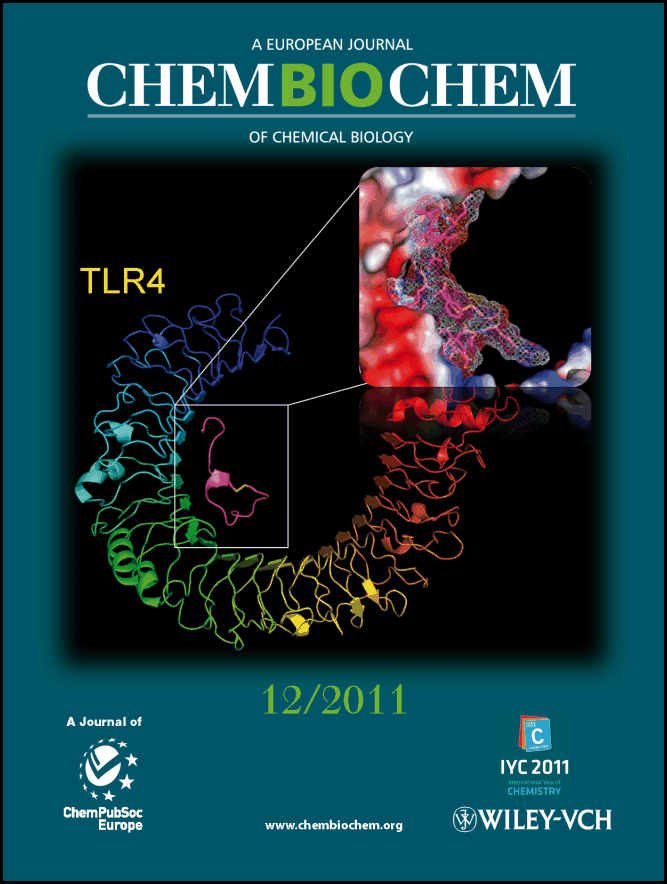
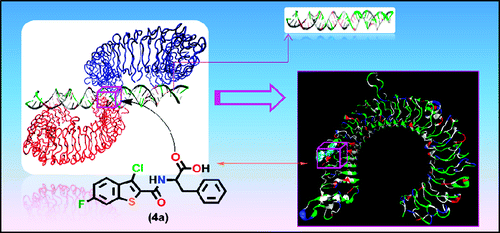
Drug Discovery: Potential Therapeutics that Inhibit Toll-like Receptors. Pain remains a significant public health issue with two-thirds of patients achieving little to no pain relief from the myriad of currently available pharmacotherapies and dosing regimens. The use of opioid pharmacotherapies produces several rewarding and reinforcing side effects, which result in their diversion to abuse settings. Glial cells have been found to play a critical role in initiating and maintaining increased nociception in response to peripheral nerve injury. The opioids-induced glial cell activation attenuates opioid-induced pain suppression and enhances the development of opioid tolerance and dependence, the drug reward, and other negative side effects such as respiratory depression. We are interested in employing structure-based drug design and high-throughput screening techniques to identify novel small-molecule inhibitors of TLR4 that regulates glial cell activation. The identified agents will potentially serve as therapeutics that can suppress opioid-dependence and tolerance. Using these methods, we also successfully identified small molecule inhibitors that disrupt the TLR3-dsRNA interaction. This is significant because the protein-RNA complex is a challenging target for small molecule probes. Our goal is to develop a generally applicable method to provide small molecule probes for the protein-protein and protein-RNA interactions of these clinically relevant TLRs.
|
||
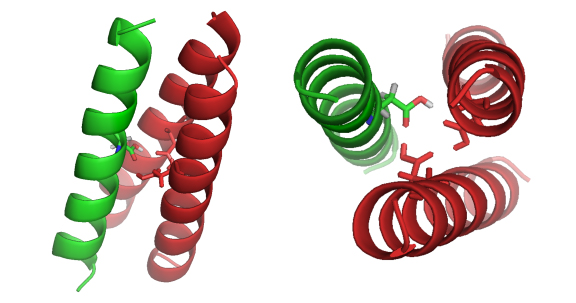
Protein Engineering: Peptides Targeting Transmembrane Domains of Proteins. Protein transmembrane domains (TMDs) regulate many pivotal biological processes, including cell signal transduction, cancer development, ion transmission, and membrane protein folding. However, the nature of molecular recognition in membranes is little understood due to the lack of available probes with high affinity and specificity. Conventional tools such as antibodies are unable to bind to the transmembrane regions of membrane proteins. A goal in our lab is to develop exogenous peptide and small-molecule agents that target transmembrane helices. Using these agents, we can study these important membrane protein-protein interactions, thereby further our understanding of molecular recognition in membranes. As a proof-of-principle, we developed novel peptide/peptidomimetic reagents to recognize the TMDs of latent membrane proteins 1 (LMP-1) found in the human Epstein-Barr herpesvirus lymphomas and syndromes. These designed peptides will be used to study TMD-mediated LMP-1 activation. The findings from these studies will lay the groundwork for the discovery of new pharmaceutical agents with which we can prevent, diagnose, and treat herpesvirus-dependent cancers. |
||
|
||
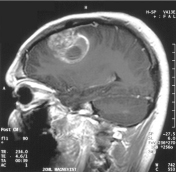
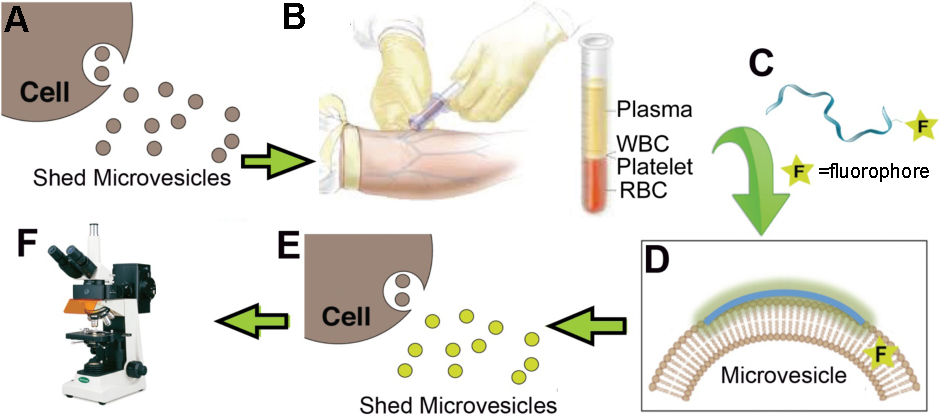
Biotechnology Development: Non-invasive Cancer Biomarkers. Elevated microvesicle (MV) shedding is an indication of cancer metastasis. Selectively sensing the highly curved membrane of MVs may provide a novel strategy for their detection, which renders the potential of developing diagnostic, prognostic, and therapeutic agents. We employed a multidisciplinary approach, utilizing our expertise in computational modeling, membrane biophysics, and cellular assay development to rationally design novel tools that sense and regulate membrane curvature and lipid components. These rationally designed agents not only help us to understand the critical protein-lipid interactions but also provide prototypes that may eventually lead to novel cancer biomarkers. Fig: Schematic of using fluorescently labeled peptide to detect shed MVs. (A) Cancerous cells shed MVs into body fluid; (B) Collection body fluid from a patient suspected to have metastatic cancers; (C) Unstructured peptide labeled with a fluorophore via a flexible linker was added into the collected body fluid specimen; (D) Curvature/lipid-sensing detects MVs by selectively binding to their highly curved surfaces or a specific lipid component, resulting in (E) the fluorescence enhancement due to the elevated hydrophobic environment of the fluorophore; (F) MV detection by fluorescently labeled curvature/lipid-sensing peptides
|
||
|